Wet Age-Related Macular Degeneration
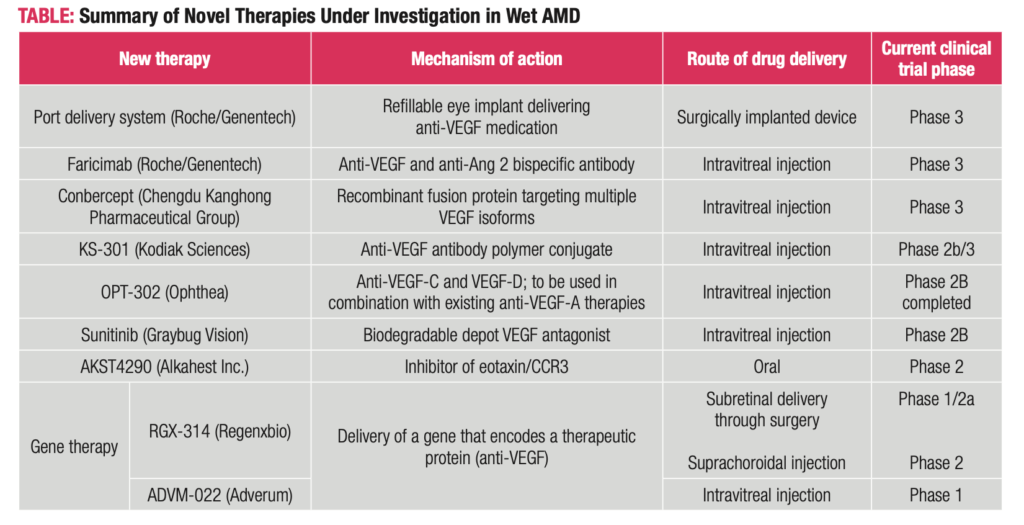
The current treatments for wet age-related macular degeneration (wAMD) inhibit a molecule called vascular endothelial growth factor (VEGF). This molecule leads to abnormal blood vessel growth and instability. Currently available anti-VEGF drugs—Avastin (Genentech), Eylea, (Regeneron), Lucentis (Genentech)—are remarkably safe and very effective for wAMD. However, with current treatments, most patients need frequent injections indefinitely. More recently, a new anti-VEGF agent, Beovu (Novartis), was FDA-approved for wAMD with suggestion of longer duration of action and reduced need for injections; however, safety concerns related to inflammation have limited widespread use of this agent. Fortunately, several clinical trials are underway to evaluate new therapies that target other pathways and/or are formulated to produce a more longer-lasting effect and, in turn, reduce treatment burden. Here, we summarize several such therapies under investigation, primarily focusing on late-stage trials (Phases 2 & 3).
Port Delivery System (Genentech): This treatment consists of a small refillable device that is implanted into the wall of the eye during a surgical procedure. The small implant stores and continuously releases anti-VEGF medication into the eye by diffusion, taking the place of frequent injections. The device can be refilled in the office. Current studies have evaluated refills every 6 months, and some early studies showed that patients could go on average about 15 months without needing a refill.1 The Port Delivery System has completed a Phase 3 clinical trial and we expect the data will be filed with the FDA soon.2
Faricimab (Genentech): Faricimab inhibits two pathways leading to abnormal blood vessel growth and leakage: It binds and inactivates a molecule called angiopoietin-2 (Ang-2) in addition to the VEGF molecule that current medications inhibit.3 By binding both molecules, faricimab may lead to improved outcomes and longer treatment duration.4 In current trials, the medication is being given up to every 4 months after a series of monthly doses. Phase 3 trials are ongoing, and we expect data in 2021.
Conbercept (Chengdu Kanghong): Conbercept is an engineered anti-VEGF that has been widely used in China since its approval there in 2013. It has been reported that conbercept may be a more potent medication because of its ability to address multiple targets, potentially resulting in a longer-lasting treatment (~3 months). Phase 3 trials in the U.S. are ongoing.5-6
KSI-301 (Kodiak Sciences): KSI-301 is a specially formulated anti-VEGF drug called an antibody polymer conjugate. The medication is expected to stick around the eye for a longer time and deliver a greater dose of medication with one injection.7 Patients in the ongoing trials are receiving treatment with the medication every 3 to 5 months, and some have gone up to 6 months before needing retreatment with an injection.8 A larger trial comparing KSI-301 to currently available medications is ongoing, and we expect to get data in 2021.
OPT-302 (Opthea): OPT-302 inhibits VEGF-C and VEGF-D, and is designed to be used in combination with the existing anti-VEGF-A therapies. The rationale is that blocking additional VEGF family members will yield a more potent anti-VEGF effect. A Phase 2 trial found that OPT-302 in combination with Lucentis was more effective than monthly Lucentis.9-10 Phase 3 trials are expected to start soon.
Sunitinib (Graybug): Sunitinib is a specially formulated depot medication that slowly dissolves over time in the eye.11 The medication acts in a slightly different way to inhibit VEGF. Because it is a depot, the medication releases over time, allowing for the possibility of extended treatment effect. In recent trials, 90% of patients were able to go 3 months without needing re-treatment, and 70% of patients were able to go to 6 months without needing retreatment. The medication is currently in a Phase 2 clinical trial.12
AKST4290 (Alkahest): AKST4290 is a small molecule inhibiting the immunomodulators playing a role in inflammation and neovascularization; two processes important in wAMD. This medication is unique from the others in that it is administered by mouth. Two small trials showed initial benefit of AKST4290 therapy in terms of vision stabilization and improvement.13-14 A larger
Phase 2 trial evaluating this medication is underway.15
Gene Therapy: Gene therapy for wet AMD is being used to create an intraocular biofactory producing a therapeutic protein. Such an approach may achieve more consistent and sustained levels of the anti-VEGF therapeutic than can be achieved with repeated injections of the medication into the eye. Thus, gene therapy has the potential to dramatically reduce treatment burden and improve outcomes by limiting the risk of period of under-treatment.
RGX-314 (RegenxBio): Regenxbio is exploring two options of delivering a gene encoding a protein similar to Lucentis directly to the space under the retina: 1) surgical administration; 2) in-office injection. Initial data demonstrate safety and reduced need for anti-VEGF injections with the surgical adminstration.16 Recently, a clinical trial to evaluate the in-office injection delivery was initiated.17
ADVM-022 (Adverum Biotechnologies): This involves an in-office (i.e., non-surgical) intravitreal injection of a gene therapy encoding the same protein as Eylea. Interim data from the ongoing early-stage trial indicates a substantial reduction in need for intravitreal injections. However, mild to moderate inflammation has been noted.18
CONCLUSION
Although existing anti-VEGF agents are very effective at treating wet AMD in the average patient, many patients require frequent injections, and some continue to lose vision despite therapy. New drugs that produce a more robust and/or longer-acting anti-VEGF effect, or that target new pathways other than VEGF, hold substantial promise for improving the vision and lives of patients with wet AMD.
Dry Age-Related Macular Degeneration
There is currently no effective treatment for the advanced form of dry AMD known as geographic atrophy (GA). Multiple clinical trials are underway studying medications that may slow the progression of GA, thereby slowing the associated retinal tissue loss and blind spot expansion experienced by patients with GA.19 Research into slowing the progression of GA has largely focused on the complement pathway of the immune system. Several complement components have been found to be important in the development or progression of GA. Two promising drugs targeting the complement system that are currently in late-stage development for GA are summarized here. Several other drugs are currently in earlier phases of clinical trials.
Pegcetacoplan (Apellis Pharmaceuticals): Pegceta-coplan (APL-2) binds and inhibits complement factor C3. After 12 months of APL-2 intravitreal injections, patients receiving APL-2 experienced a reduction in the growth of GA in a large study.20 Two Phase 3 clinical trials are currently ongoing, and we expect data in 2021.
Zimura (Iveric Bio): Zimura (avacincaptad pegol) is a complement C5 inhibitor that is given via intravitreal injection. In a recent study, patients who received Zimura experienced a significant reduction in GA growth compared to the control group.21 Another large study involving Zimura is now underway.


For a printable version of this PDF, click here.
References
- Campochiaro PA, et al. Ophthalmology. 2019;126(8):1141-1154.
- Clinicaltrials.gov . Available at: https://clinicaltrials.gov/ct2/show/NCT03677934 . Accessed Aug. 28, 2020.
- Hackett SF, et al. J Cell Physiol. 2002;192(2):182-187.
- Khanani AM. Paper presented at the Retina Subspecialty Day, American Academy of Ophthalmology Meeting, Chicago, Illinois, October 26, 2018.
- Clinicaltrials.gov . Available at: https://clinicaltrials.gov/ct2/show/NCT03577899 . Accessed Aug. 27, 2020.
- Clinicaltrials.gov . Available at: https://clinicaltrials.gov/ct2/show/NCT03630952 . Accessed Aug. 27, 2020.
- Wykoff CC. Presented at AAO Subspecialty Day Retina 2019; October 11, 2019; San Francisco, CA.
- Available at: https://clinicaltrials.gov/ct2/show/NCT04049266 . Accessed August 28, 2020.
- Jackson T. Paper presentation at EURETINA Congress; September 5, 2019; Paris, France.
- Opthea. Available at: https://bit.ly/31UNtVN . Accessed Aug. 27, 2020.
- Bhatt P, et al. AAPS PharmSciTech. 2019;20(7):281.
- Graybug Vision. Available at: https://bit.ly/352iTM3 . Accessed Aug 27, 2020.
- Ois.net . Available at: https://bit.ly/3jJiVMR . Accessed Aug 27, 2020.
- Stewart MW. Paper presentation at AAO Annual Meeting; Oct. 11-15, 2019; San Francisco, CA, USA.
- Clinicaltrials.gov . Available at: https://clinicaltrials.gov/ct2/show/NCT04331730 . Accessed Aug 27, 2020.
- BioSpace.com . Available at: https://bit.ly/3jJRqCU . Accessed Aug 27, 2020.
- Clinicaltrials.gov . Available at: https://clinicaltrials.gov/ct2/show/NCT04514653 . Accessed Aug 27 2020.
- GlobeNewswire. Available at: https://bit.ly/2QQXixJ . Accessed Aug 27, 2020.
- Fleckenstein M, et al. Ophthalmology. 2018;125(3):369-390.
- Liao DS, Grossi FV, et al. Ophthalmology. 2020;127(2):186-195.
- IVERIC Bio. Available at: https://bit.ly/3lLJHpv . Accessed Sept. 1, 2020.